
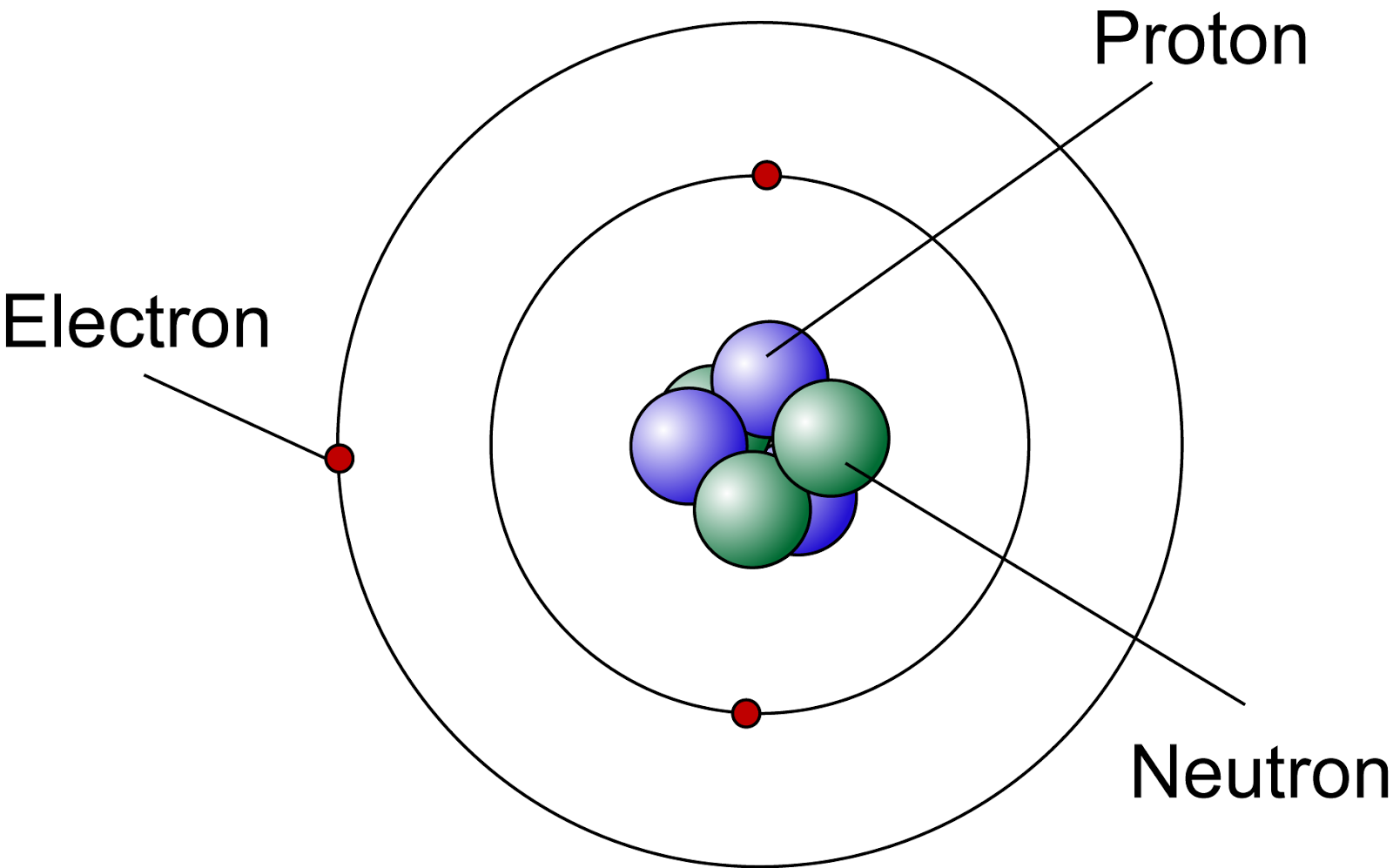
By treating electrons as particles, the Bohr model implied that a hydrogen atom would be essentially two dimensional, In the Bohr model of the atom, electrons are described as having circular, planar orbitals around a central nucleus. In this question, we are given five statements about the Bohr model and need to identify which of them is Discoveries in physics and the formulation of quantum mechanics highlighted inadequacies in the Bohr model The Bohr model of the atom was at one point the best description of an atom.

It explains the line emission spectrum of the hydrogen atom only.Electrons within atoms can only occupy quantized energy levels.
 It is possible to precisely determine the position and momentum of an electron simultaneously. Electrons are only considered as particles and not as waves. Electrons move around the nucleus in circular, planar orbits. Which of the following is not a limitation of the Bohr model of the atom? The name we give to this concept is wave–particle duality.Įxample 1: Identifying Which Statement Is Not a Limitation of the Bohr Model of the Atom However, in general, we only observe wave-like behavior from particles moving at speeds It turns out that everything we call a particle can be described in wave-like ways, and everything we call a wave can beĭescribed in particle-like ways. De Broglie demonstrated that electrons had wave-like properties as well as particle-like properties. Louis de Broglie provided one of the most crucial insights that redefined how we imagine how electrons behave. Werner Heisenberg’s work on the uncertainty principle was part of a larger body of work that expanded how we describe Uncertainty principle, so the Bohr model must be incorrect in some way. The Bohr model treats electrons purely as particles that orbit in a predictable way. Significant for subatomic particles, or larger objects moving at very high speeds. In simple terms, we can either know where a particle is or how fast it is going (and in what direction). The more accurately you determine a particle’s position in space, the more uncertain your measurement of its momentum Definition: Heisenberg’s Uncertainty Principle
It is possible to precisely determine the position and momentum of an electron simultaneously. Electrons are only considered as particles and not as waves. Electrons move around the nucleus in circular, planar orbits. Which of the following is not a limitation of the Bohr model of the atom? The name we give to this concept is wave–particle duality.Įxample 1: Identifying Which Statement Is Not a Limitation of the Bohr Model of the Atom However, in general, we only observe wave-like behavior from particles moving at speeds It turns out that everything we call a particle can be described in wave-like ways, and everything we call a wave can beĭescribed in particle-like ways. De Broglie demonstrated that electrons had wave-like properties as well as particle-like properties. Louis de Broglie provided one of the most crucial insights that redefined how we imagine how electrons behave. Werner Heisenberg’s work on the uncertainty principle was part of a larger body of work that expanded how we describe Uncertainty principle, so the Bohr model must be incorrect in some way. The Bohr model treats electrons purely as particles that orbit in a predictable way. Significant for subatomic particles, or larger objects moving at very high speeds. In simple terms, we can either know where a particle is or how fast it is going (and in what direction). The more accurately you determine a particle’s position in space, the more uncertain your measurement of its momentum Definition: Heisenberg’s Uncertainty Principle







 0 kommentar(er)
0 kommentar(er)
